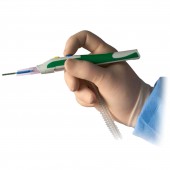
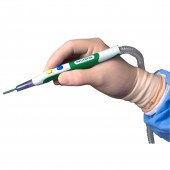
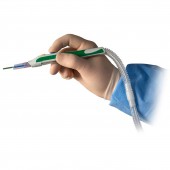
ZIP Pen® Electrosurgical Smoke Evacuation Pen by Megadyne Medical Products |
Home > Winners > #42871 |
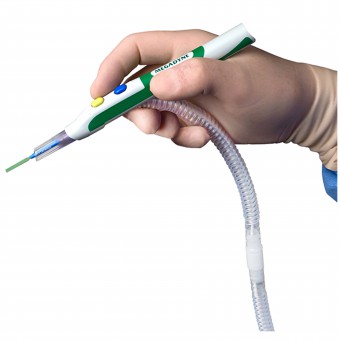 |
|
||||
| DESIGN DETAILS | |||||
| DESIGN NAME: ZIP Pen® PRIMARY FUNCTION: Electrosurgical Smoke Evacuation Pen INSPIRATION: After years of exposure to surgical smoke while observing cases, we had a desire to improve the acceptability of surgical smoke capture in order to protect Surgeons, Staff, and Patients. The ergonomic advantages of ZIP Pen eliminate torque created by the attached tubing which makes traditional smoke evacuation pencils difficult and tiring to use. Surgeon comfort is critical to acceptance of a device that improves environmental quality. This design eliminates user objections, increases use, and improves environmental conditions in the O.R. UNIQUE PROPERTIES / PROJECT DESCRIPTION: ZIP Pen's unique and innovative design allows Surgeons to experience comfortable and precise electrosurgery while minimizing the hazards of exposure to surgical smoke. Unlike traditional smoke evacuation devices, ZIP Pen offers Surgeons customized ergonomic adjustability for maximum comfort and functionality. ZIP Pen is the only surgical smoke pen with the ability to remove 100 percent of tubing torque from the back of the pencil. OPERATION / FLOW / INTERACTION: ZIP Pen is used to apply electrical energy to tissue in order to cut and coag (stop bleeding) during surgery. Smoke is created by this process which contains harmful substances and may contain active viruses. ZIP Pen helps collect this smoke to protect people in the O.R. and the design provides customized adjustment of the tubing to improve usability and eliminate user objections. ZIP Pen design features result in improved compliance and environmental safety for Surgeons, Staff and Patients. PROJECT DURATION AND LOCATION: The ZIP Pen project was officially started in Sept 2012 with completion of all regulatory requirements and product release in July of 2015. All Design Engineering work was conducted at Megadyne in Draper Utah USA. FITS BEST INTO CATEGORY: Medical Devices and Medical Equipment Design |
PRODUCTION / REALIZATION TECHNOLOGY: Simply holding a traditional smoke pen allows easy identification of user objections; they feel heavy and difficult to control. Design iterations combined with rapid prototyping (3D printing) helped identify the perfect balance of tubing location, handle shape, and button location. Development of a fixture (also 3D printed) helped validate the elimination of torque forces. This process allowed early design refinement which improved time to production and minimized tooling adjustments. SPECIFICATIONS / TECHNICAL PROPERTIES: ZIP Pen handle dimensions are approximately 174 mm long x 16 mm wide x 20 mm high. The handle has a slight taper from the buttons to the proximal end for ergonomic handling, and a more rapid taper from the buttons to the distal end to facilitate visibility of the tip. Tubing comes in two lengths of approximately 3 m or 4.5 m to allow flexibility in placement of Electrosurgical units and Smoke Evac units in the surgical suite. All materials are ROHS compliant and are fully tested for biocompatibility. TAGS: Electrosurgery, Smoke Evacuation, ZIP Pen, Megadyne, E-Z Clean, The Electrosurgical Authority RESEARCH ABSTRACT: Background research consisted of identifying the causes for Surgeon rejection of traditional smoke evac pens. Once identified, an iterative process of 3D printed designs and Surgeon evaluation was used, combined with torque measurements to insure the lowest possible values. While the design is simple, it is not simplistic, and many who have seen it say, "why didn't I think of that". ZIP Pen can have a huge impact in the surgical suite because it increases compliance with smoke evacuation methods for a safer surgical environment. CHALLENGE: Design for assembly principles were used to minimize difficulties associated with the new design of ZIP Pen. Electrical connections within the handle had to be made between multiple components without fail. Ensuring these connections were made appropriately every time required careful design, assembly, and validation efforts. A snap together design that would never be exposed to excessive applied forces during use was also a challenge that was successfully met. ADDED DATE: 2015-09-25 18:31:48 TEAM MEMBERS (9) : V.P. of Engineering: Paul Borgmeier, Marketing Product Manager: Cameron Creek, President/C.E.O: Rob Farnsworth, Sr. Designer: Chad Frampton, Sr. Project Engineer: Mark Glassett, R&D Engineer/Inventor: Darcy Greep, V.P. of Marketing: Mike Hintze, Chief Medical Officer: Ryan Lewis and Marketing Product Manager: Jill Skoczen IMAGE CREDITS: Megadyne Medical Products, 2015. PATENTS/COPYRIGHTS: Patented, 8,211,103, Megadyne Medical Products, 2012, US Patented, 8,882,767, Megadyne Medical Products, 2014, US Patented, 8,882,768, Megadyne Medical Products, 2014, US Patented, D709,196, Megadyne Medical Products, 2014, US Other US, International, and Foreign Patents Pending |
||||
| Visit the following page to learn more: http://www.megadyne.com | |||||
| AWARD DETAILS | |
 |
Zip Pen® Electrosurgical Smoke Evacuation Pen by Megadyne Medical Products is Winner in Medical Devices and Medical Equipment Design Category, 2015 - 2016.· Press Members: Login or Register to request an exclusive interview with Megadyne Medical Products. · Click here to register inorder to view the profile and other works by Megadyne Medical Products. |
| SOCIAL |
| + Add to Likes / Favorites | Send to My Email | Comment | Testimonials | View Press-Release | Press Kit |