NEXThaler® Dry Powder Inhaler (DPI) by Chiesi Farmaceutici S.p.a. |
Home > Winners > #26752 |
 |
|
||||
| DESIGN DETAILS | |||||
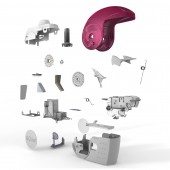
| DESIGN NAME: NEXThaler® PRIMARY FUNCTION: Dry Powder Inhaler (DPI) INSPIRATION: Despite the availability of many pharmacological interventions for the treatment of asthma, many patients fail to manage their asthma properly (Elliot, 2006), leading to increased mortality (McCowan et al., 2005; Holgate et al., 2008; Murphy, 2010). Previous literature has strongly suggested that optimal device characteristics and patient preference for a device can contribute to greater asthma control (Price et al., 2003; Chyrstyn, 2007; Chyrstyn & Price, 2009; see review Anderson, 2005). UNIQUE PROPERTIES / PROJECT DESCRIPTION: This new dry powder inhaler contains up to 120 doses of medicament, an innovative full dose feedback system incorporating a novel breath-actuated mechanism: a click is heard and a dose counter decrements by one count, only after effective full dose delivery. The device acts both as protective container closure system for the drug product, reducing absorption of moisture by the drug substance, and as a drug delivery device for the medicinal product. OPERATION / FLOW / INTERACTION: The operating sequence requires only three step: open, inhale and close. There is a breath actuated digital dose counter to display the number of doses remaining. The advantages over existing devices are: Simplest possible sequence to reduce training time and maximise patient compliance (less operating actions than existing devices on the market). Only actuations taken are inhaled and displayed on the dose counter to reduce wastage and give a true indication of the number of doses inhaled. PROJECT DURATION AND LOCATION: Chiesi Farmaceutici wanted to develop a successor to its inhaler named PULVINAL(R), already marketed in some European countries, for both its approved drug substances and drug substances in development. In 2001 Chiesi started the project; the scope of the project was to develop a truly modern inhalation product that offered Chiesi a robust technology platform for future drug product developments. The device coupled with a new drug product was submitted in 2011 and the product was approved in 2012. FITS BEST INTO CATEGORY: Medical Devices and Medical Equipment Design |
PRODUCTION / REALIZATION TECHNOLOGY: NEXThaler(R) consists of different parts made of polymeric and metal materials. The device comprises two functional groups of components coupled together. The Dosing group meters the drug volumetrically from a bulk reservoir and permits de-aggregation and delivery of the product to the patient. The Counting group includes the breath actuated mechanism, which activates the dosing group under a certain air flow allowing the dose to be taken, and a dose counter decrements each time it is activated. SPECIFICATIONS / TECHNICAL PROPERTIES: 78,0 mm x 30,0 mm x 58,0 mm TAGS: Easy to use, DPI, Open inhale close, full dose feedback system RESEARCH ABSTRACT: A recent study shows that NEXThaler(R) is more effective, efficient and preferred to use and own when compared to two leading DPI products on the market (Linnane et al. 2012). The study was conducted on 66 patients. In terms of effectiveness and efficiency, patients made significantly more successful uses and fewer task step failures, were quickest to set-up and also quickest to read the IFU for NEXThaler (R) compared to the Diskus (R) and Turbuhaler (R). CHALLENGE: It was challenging to combine both ease of use for the intended population and effective therapeutic results in one product. The challenge for the designers was to combine many different complex mechanical features (breath activated drug delivery mechanism, robust dose counting, full dose feedback) in a device that appears simple and intuitive to use (open, inhale & close). ADDED DATE: 2012-09-28 06:55:13 TEAM MEMBERS (13) : Alice Campanini, Patrick Gerard Linnane, Gaetano Brambilla, Lorenzo Zuccheri, Irene Pasquali, Maria Chiara Taverna, Claudio Piazza, Cambridge Consultants Limited, Daniela Cocconi, Riccardo Villani , Rossella Musa, Annamaria Cantarelli and Paolo Patri IMAGE CREDITS: Chiesi Farmaceutici S.p.a., 2012. |
||||
| Visit the following page to learn more: http://www.chiesigroup.com | |||||
| AWARD DETAILS | |
 |
Nexthaler® Dry Powder Inhaler (dpi) by Chiesi Farmaceutici S.p.a is Winner in Medical Devices and Medical Equipment Design Category, 2012 - 2013.· Press Members: Login or Register to request an exclusive interview with Chiesi Farmaceutici S.p.a.. · Click here to register inorder to view the profile and other works by Chiesi Farmaceutici S.p.a.. |
| SOCIAL |
| + Add to Likes / Favorites | Send to My Email | Comment | Testimonials | View Press-Release | Press Kit |