Roboangio Interventional Robotic System by Wenyong Ren and Weinan Yang - Abrobo |
Home > Winners > #159095 |
 |
|
||||
| DESIGN DETAILS | |||||
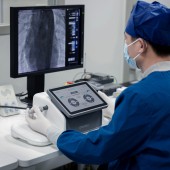
| DESIGN NAME: Roboangio PRIMARY FUNCTION: Interventional Robotic System INSPIRATION: The incidence of vascular diseases is on the rise globally. However, to treat vascular diseases via interventional surgeries, a physician faced massive radiation exposure and manual errors. Numerous patients could not access an effective treatment. The design team cooperated with clinical experts to gain original insights, through which this system was invented to change the traditional approach. UNIQUE PROPERTIES / PROJECT DESCRIPTION: The Roboangio is a new interventional robotic system intended for use in the remote delivery and manipulation of guidewires and catheters during vascular interventional surgeries. With the design of a master-slave control solution, it enables physicians to stay away from radiation environment while preserving complete control. The design brings ease to physicians and safety to patients through a combination of intuitive interaction and robotic precision. OPERATION / FLOW / INTERACTION: The design team believes that its successful application relies on how well the technology can fit into existing surgical environments and techniques. The adjustable gantry can be easily attach to a surgical table when needed and removed when it is not. The control console has two operating rods that reproduce the feel of traditional practices, so physicians can use their well-honed skills when performing interventional surgery with robotic technology. PROJECT DURATION AND LOCATION: Development of this project began in March 2021. Clinical trial of the product concluded in June 2023. Released to public in October 2023. Designed in Shenzhen, Guangdong, China. FITS BEST INTO CATEGORY: Medical Devices and Medical Equipment Design |
PRODUCTION / REALIZATION TECHNOLOGY: Electromechanical Integration, Control Algorithms, Radiation-shielded Lead Panel and Lead Glass, Biocompatible Injection-molded Plastics, Precision Machined Aluminium, Ethylene Oxide Sterilization SPECIFICATIONS / TECHNICAL PROPERTIES: Robotic Drive: 1430mm length X 365mm width X 305mm height, Control Console: 540mm length X 250mm width X 220mm height, Shielded Workstation: 1140mm length X 845mm width X 1410mm height, Adjustable Gantry: 1060mm length x 305mm width x 660mm height, Transit Trolley: 1014mm length x 817mm width x 1020mm height TAGS: Robotics, Surgery, Vascular, Endovascular, Intervention, Radiation, Angiography, Medical Device RESEARCH ABSTRACT: Research for this project went through many stages. Following the process of developing a medical device for approval by the NMPA is critical to this project. This involved a series of strategic R&D activities proceeding from Inputs, Outputs, Validation to Verification. Data was analyzed through experiments of MVP, formative and summative tests as well as clinical trials. Our design team worked with clinical experts, ensuring that needs were well received and effectively carried to the design. CHALLENGE: For project of this kind, challenges existed in almost all aspects. During the creative process, one of the hardest parts is to objectively receive voices from various perspectives and still hold onto a clear vision of how to empower interventional surgery with robotic technology. Through persistent efforts, this project has overcame obstacles involved multi-disciplinary electromechanical integration, complex control algorithms as well as aseptic manufacturing techniques. ADDED DATE: 2024-02-26 08:06:48 TEAM MEMBERS (4) : Wenyong Ren, Weinan Yang, Zanzhong Xia and Hangye Xu IMAGE CREDITS: Weinan Yang, Ruiming Wu, Yunong Zeng, Zhihao Hu, Zanzhong Xia, Hangye Xu, Roboangio, 2024. PATENTS/COPYRIGHTS: Patent No: CN113749774B, CN115245387B, CN114191096B, CN114521971B, CN114469354B, CN113633389B, CN113729965B, CN113749782B, CN113749779B, CN113796965B, CN114391962B, CN114191079A, CN114391950A, CN114391965A, CN307987004S, CN307907111S, CN307672296S, CN307947684S, CN307907110S, CN307683213S |
||||
| Visit the following page to learn more: https://cutt.ly/Rw1f29lf | |||||
| AWARD DETAILS | |
 |
Roboangio Interventional Robotic System by Wenyong Ren and Weinan Yang-Abrobo is Winner in Medical Devices and Medical Equipment Design Category, 2023 - 2024.· Press Members: Login or Register to request an exclusive interview with Wenyong Ren and Weinan Yang - Abrobo. · Click here to register inorder to view the profile and other works by Wenyong Ren and Weinan Yang - Abrobo. |
| SOCIAL |
| + Add to Likes / Favorites | Send to My Email | Comment | Testimonials | View Press-Release | Press Kit |